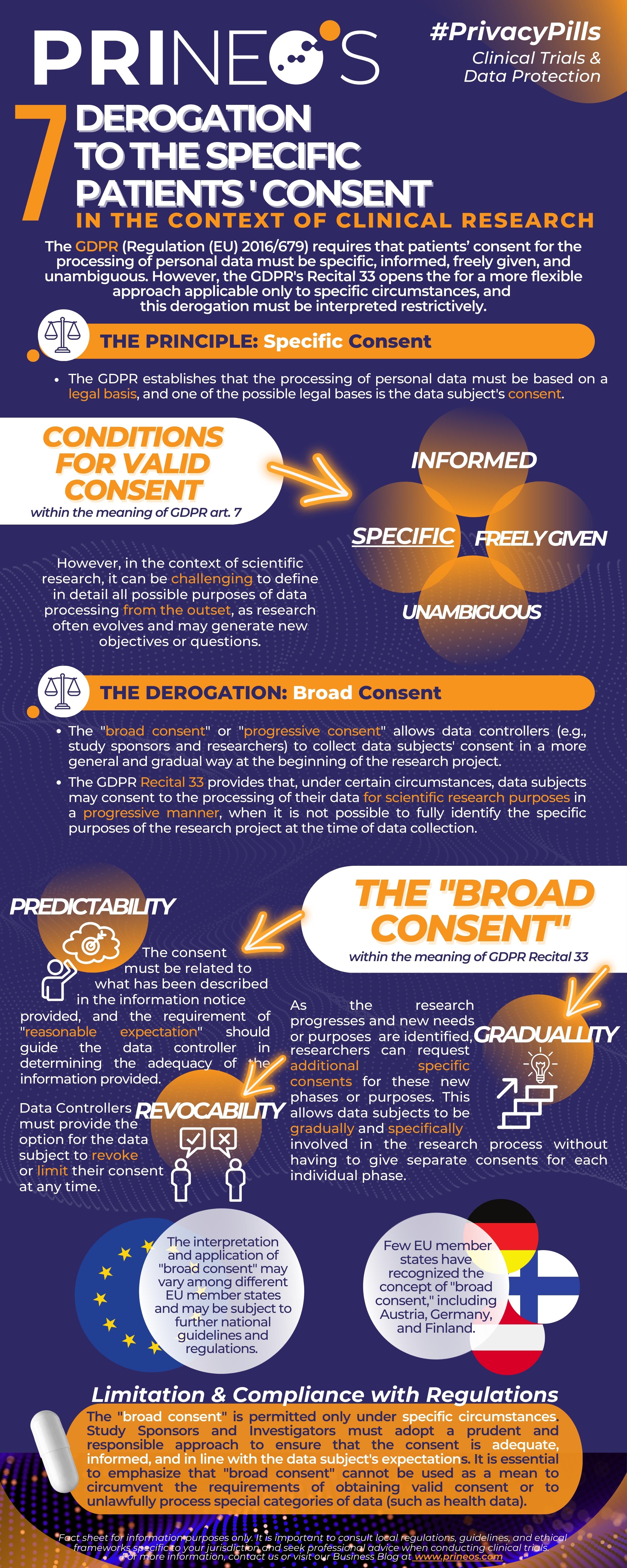
This week's Privacy Pill will focus on a specific concept that carves out a privileged position for scientific research: so-called "broad consent", also known as progressive consent.
At the core of the GDPR lies the principle of specific consent - a crucial cornerstone for data processing. But what happens when the objectives of scientific research are not entirely clear from the outset? How can we strike a balance between the need for precise consent and the evolving nature of research?
In this 7th Fact Sheet, we delve into the intricacies of "broad consent", exploring how it allows data controllers (e.g., sponsors and investigators), to seek more general consent at the outset of research projects. This progressive approach gives subjects the flexibility to participate in the research journey while ensuring that their privacy is respected.