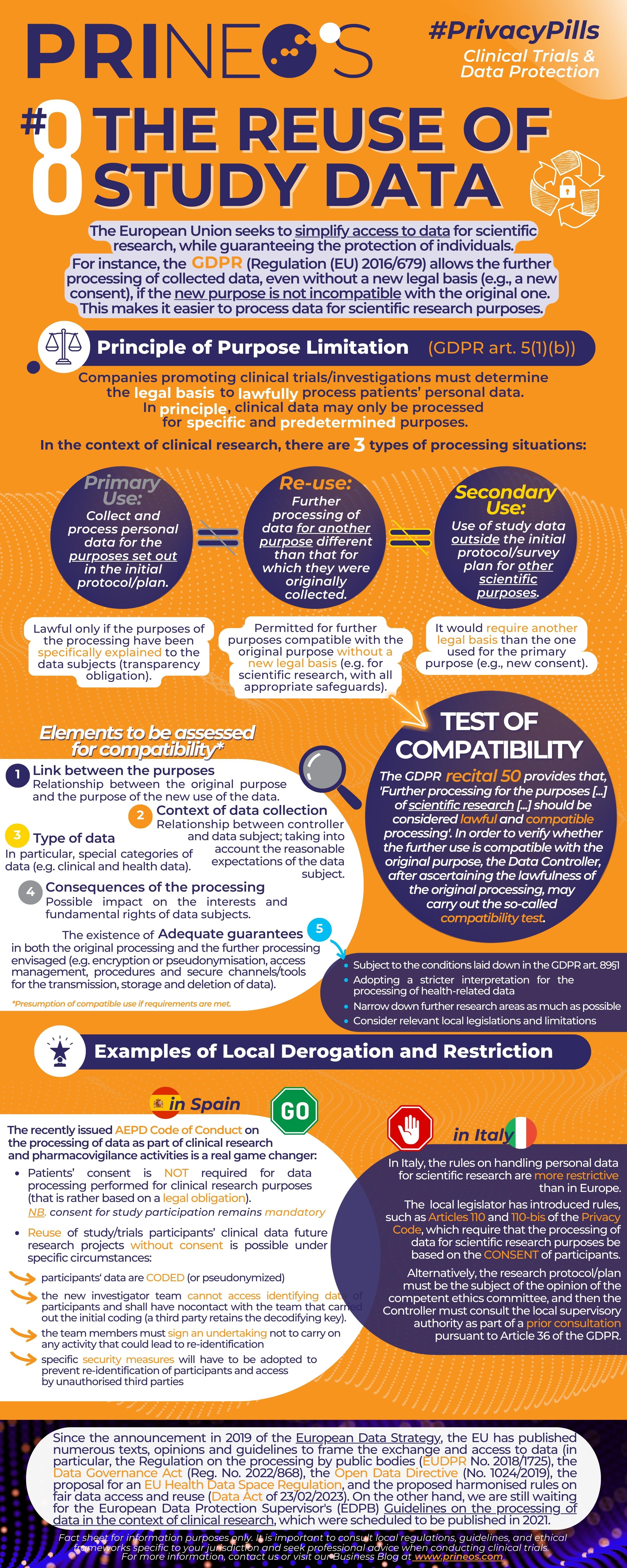
In Europe, there's a growing recognition of the immense value locked within clinical trial data. These data repositories hold a treasure trove of insights, not only for the initial research but also for broader scientific endeavors.
The GDPR (General Data Protection Regulation) provides a robust framework for data protection, even when it comes to the reuse of clinical trial participants' data. While respecting individuals' privacy rights remains paramount, GDPR's provisions also allow for the responsible and ethical reuse of this data for further scientific purposes.
Researchers are now exploring innovative ways to harness this data while ensuring strict adherence to GDPR guidelines. It's an exciting time for science as we strike that delicate balance between data privacy and the pursuit of knowledge that can benefit us all.
Scientific research is crucial for progress and the development of new solutions and treatments. However, in Italy, there are some significant challenges when it comes to managing personal data for scientific research purposes.
It's essential to have a global perspective on how data is managed for scientific research and seek solutions that can meet international standards without compromising data privacy and security.